Neuropixels, Plainly Explained
A 1-Minute Overview of Neuropixels
What if you could listen to hundreds of brain cells at once and still keep the data trustworthy and easy to share? This is what Neuropixels makes possible and how the community turns those signals into reliable results.
Quick hits: what Neuropixels make possible
Neuropixels are silicon probes that continuously detect tiny voltage changes from nearby neurons. Neuropixels probes, first released in 2017, set a new gold standard for large-scale electrophysiology [1]. The goal was simple to state and hard to do: record many single neurons in freely behaving animals with light, flexible hardware and many recording channels. Earlier probe stacks, such as passive NeuroNexus shanks with INTAN headstages, offered only tens of channels and relied on long analog cables that picked up electrical noise.
Neuropixels take a different path. Using complementary metal oxide semiconductor (CMOS) fabrication, the probe integrates amplification, multiplexing, and digitization on the device itself. Placing the electronics next to the recording sites shortens analog paths, reduces noise, and enables hundreds of addressable channels. This was not practical earlier because fabrication yields on long slender shanks were low, power and heat budgets were tight, and packaging had to remain biocompatible. Process advances and coordinated investment made it feasible. The result was a step change in experimental capability: stable, simultaneous recordings from hundreds of neurons across multiple brain regions in freely moving animals [1].
Adoption is broad. Our conservative estimate is that more than 850 laboratories have used Neuropixels since 2017*.
Who built it
The Neuropixels project is an international collaboration led by IMEC (Interuniversity Microelectronics Centre, Belgium) with partners including the Wellcome Trust, Howard Hughes Medical Institute (HHMI) Janelia Research Campus (Timothy Harris and colleagues), University College London (Matteo Carandini, Nicholas Steinmetz, and collaborators), the Allen Institute for Brain Science (Christof Koch and colleagues), Gatsby Charitable Foundation, the Sainsbury Wellcome Centre (John O'Keefe and colleagues), among others acknowledged in the original publications [1-3]. Their collaboration transformed what was once a technological aspiration into an accessible community resource. Neuropixels 2.0, co-developed with Cambridge NeuroTech, built upon this foundation with improved reusability and chronic stability [4,5].
From 1.0 to Opto: The Probe Line-Up
The development of Neuropixels has progressed through a series of significant releases, each enhancing its capabilities and resolution. This evolution highlights the ongoing advancements in neural recording technology.
Most Neuropixels models allow you to select up to 384 channels to record simultaneously from many more physical sites on the shank. The variants below differ mainly in site geometry, density, shank layout, and added capabilities (primate length, ultra-dense mapping, or built-in light for optogenetics).
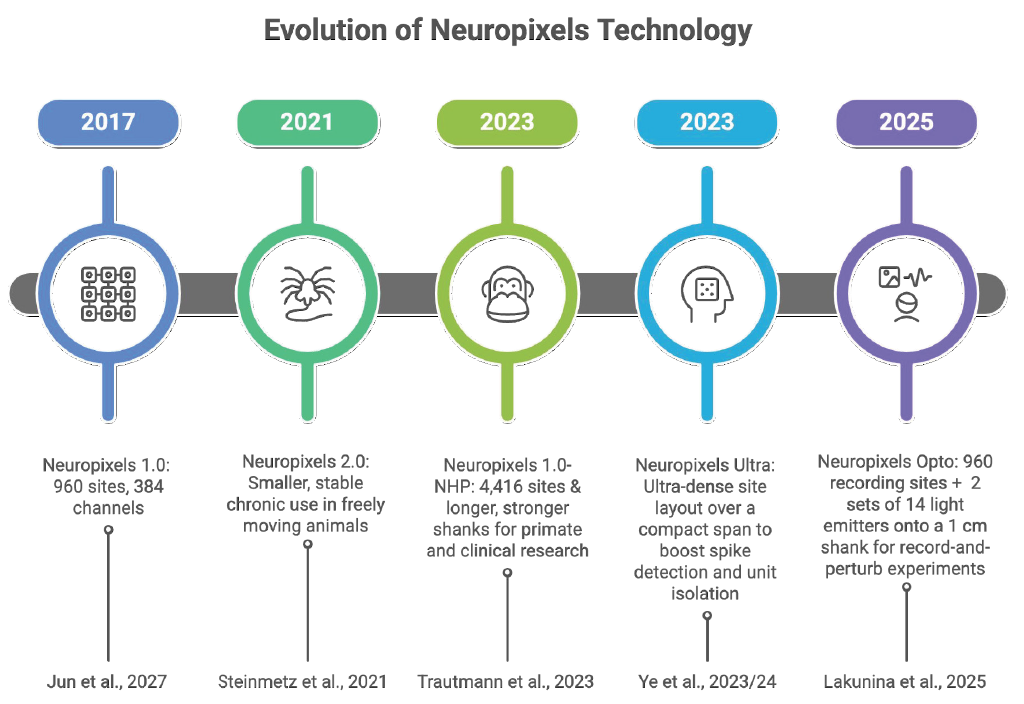
Since its debut in 2017, the Neuropixels series has revolutionized neuroscience research. The original Neuropixels 1.0 featured 960 recording sites setting a new standard for high-density neural data collection in rodents. Building on that success, Neuropixels 2.0 arrived in 2021 with 1,280 recording sites per shank, ideal for chronic experiments with freely moving animals. By 2023, the lineup expanded further to include Neuropixels NHP for primates and clinical research. The same year, Neuropixels Ultra dramatically increased spatial resolution by pushing site density even higher for more precise spike detection and cell-type identification. Most exciting, the 2025 introduction of Neuropixels Opto combines electrophysiology with neurophotonics, enabling simultaneous electrical recordings and optical manipulation of neuronal activity through optogenetics.
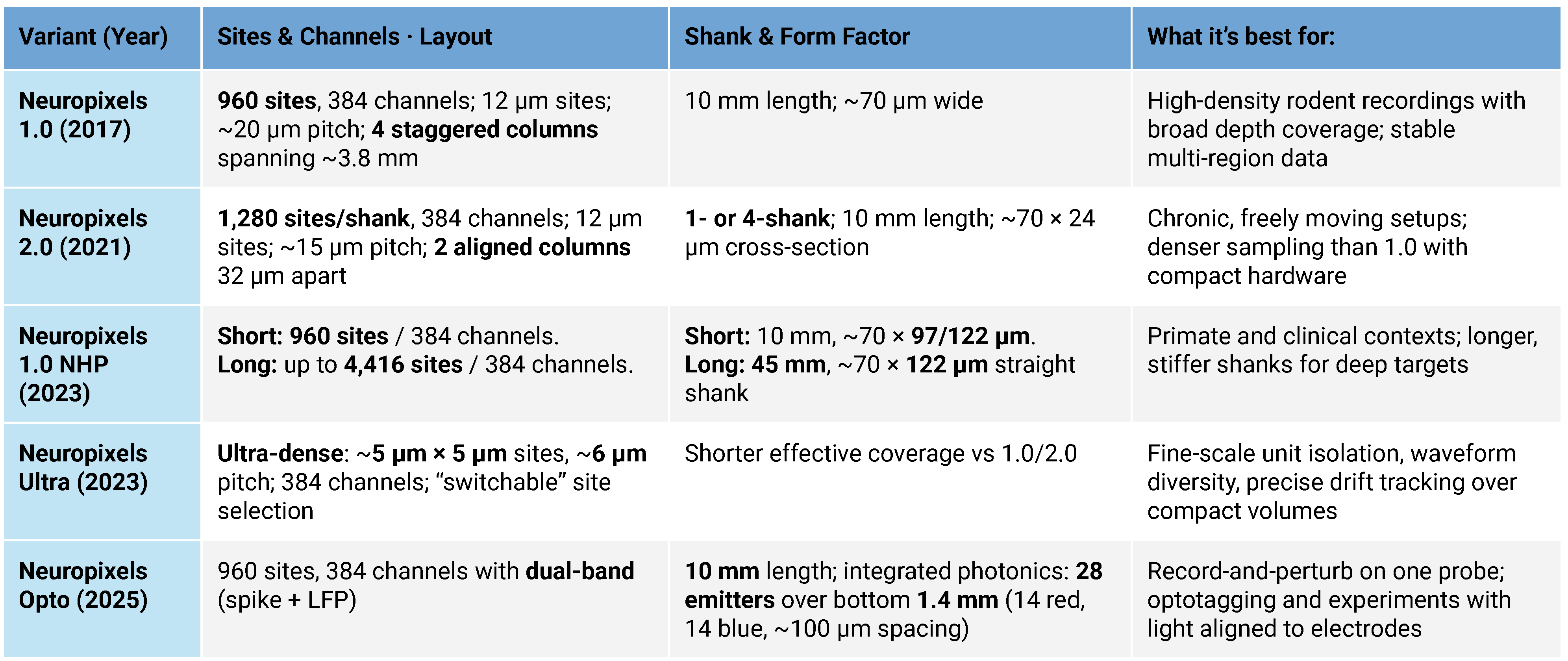
These innovations represent a significant leap forward in neural research, evolving from small-scale rodent studies to detailed, large-scale analyses of the brain system. This includes ultra-high-resolution mapping and multimodal data integration, opening an exciting new chapter in neural exploration.
The Three-Step Guide to Choosing a Probe
Different probe designs serve different needs. Your choice should follow the question, species, surgery plan, recording duration, and budget.
Neuropixels integrates on-probe CMOS electronics with very high site counts per shank. Helpful for dense, stable, multi-region recordings in freely moving animals.
NeuroNexus offers a broad range of passive silicon probes in many geometries for acute and chronic in vivo recordings. Probes pair with external or headstage electronics, which keep them slender and flexible in configuration. Compared with Neuropixels, per-shank site counts are lower, with 32 sites per shank, and digitization occurs off-probe. Useful when you need custom layouts or thinner shanks.
INTAN Technologies offers low-noise amplification and digitization on the headstage used across many passive probes, including NeuroNexus and Cambridge NeuroTech. Adds headstage weight and analog cable length compared with on-probe designs. Flexible and cost-aware.
Cambridge NeuroTech focuses on chronic-focused silicon passive probes and implant hardware; partner on Neuropixels 2.0. Pairs with external electronics; their strengths include chronic implant hardware and diverse probe geometries. Strong for long-term stability and surgical accessories.
NeuroSeeker (EU-funded project) was an earlier initiative involving the Harris Lab at Janelia, IMEC, and UCL that advanced dense CMOS concepts that informed today’s designs.
The Masmanidis Lab at UCLA creates silicon probes with custom geometries, often distributed through collaborations with NeuroNexus or Cambridge NeuroTech. Good for specialised layouts.
Diagnostic Biochips builds high-density, small-form-factor probes designed for chronic rodent work. Emphasizes compact headstages and implant practicality.
How to choose:
- Need highest per-shank site density and fewer external cables? Consider on-probe-CMOS designs.
- Need unusual geometries or ultra-thin shanks? Consider passive silicon lines with external headstages.
- Long-term chronic implant with specific surgical hardware? Choose vendors with mature chronic ecosystems (e.g., Cambridge NeuroTech, Diagnostic Biochips) and proven accessories.
From Acquisition to Sorting: The Open Toolchain at a Glance
Open tools made Neuropixels practical at scale:
- SpikeGLX for high-performance data acquisition software optimized for Neuropixels. It enables stable, low-latency streaming of hundreds of channels. Developed by Bill Karsh (HHMI Janelia) and colleagues.
- Open Ephys for a modular, open-source acquisition hardware and software for real-time electrophysiology. While compatible with multiple probes, it has been widely adopted for Neuropixels acquisition. Created by Josh Siegle (Allen Institute) and Jakob Voigts (MIT, Open Ephys company).
- Kilosort for fast, GPU-based spike sorting tuned for dense data produced by Neuropixels. Developed by Marius Pachitariu and collaborators (originally at UCL and Janelia).
- SpikeInterface for standardized preprocessing, spike sorting, validation, and comparison across multiple algorithms, including Kilosort. It integrates smoothly into Neuropixels workflows. Led by Alessio Buccino and Cyrille Rossant with an international community.
- The Allen Institute Ecephys pipeline for quality metrics and alignment practices, forming the backbone of the Allen Brain Observatory’s large-scale projects. Maintained by Josh Siegle and the Allen Institute team.
Together, these tools create an ecosystem that supports the acquisition, processing, and analysis of Neuropixels data with rigor and reproducibility. Their open-source nature has ensured broad adoption across labs worldwide, cementing Neuropixels as the foundation for modern systems neuroscience.
DataJoint in Action: What You Get
Running Neuropixels at scale is a data complexity problem, especially when done in multimodal experiments synchronizing other instruments.
DataJoint directly addresses the challenge of managing and analyzing terabyte-scale Neuropixels datasets in combination with other instruments as reproducible, cloud-ready workflows.
Open-Source DataJoint Pipeline at hand
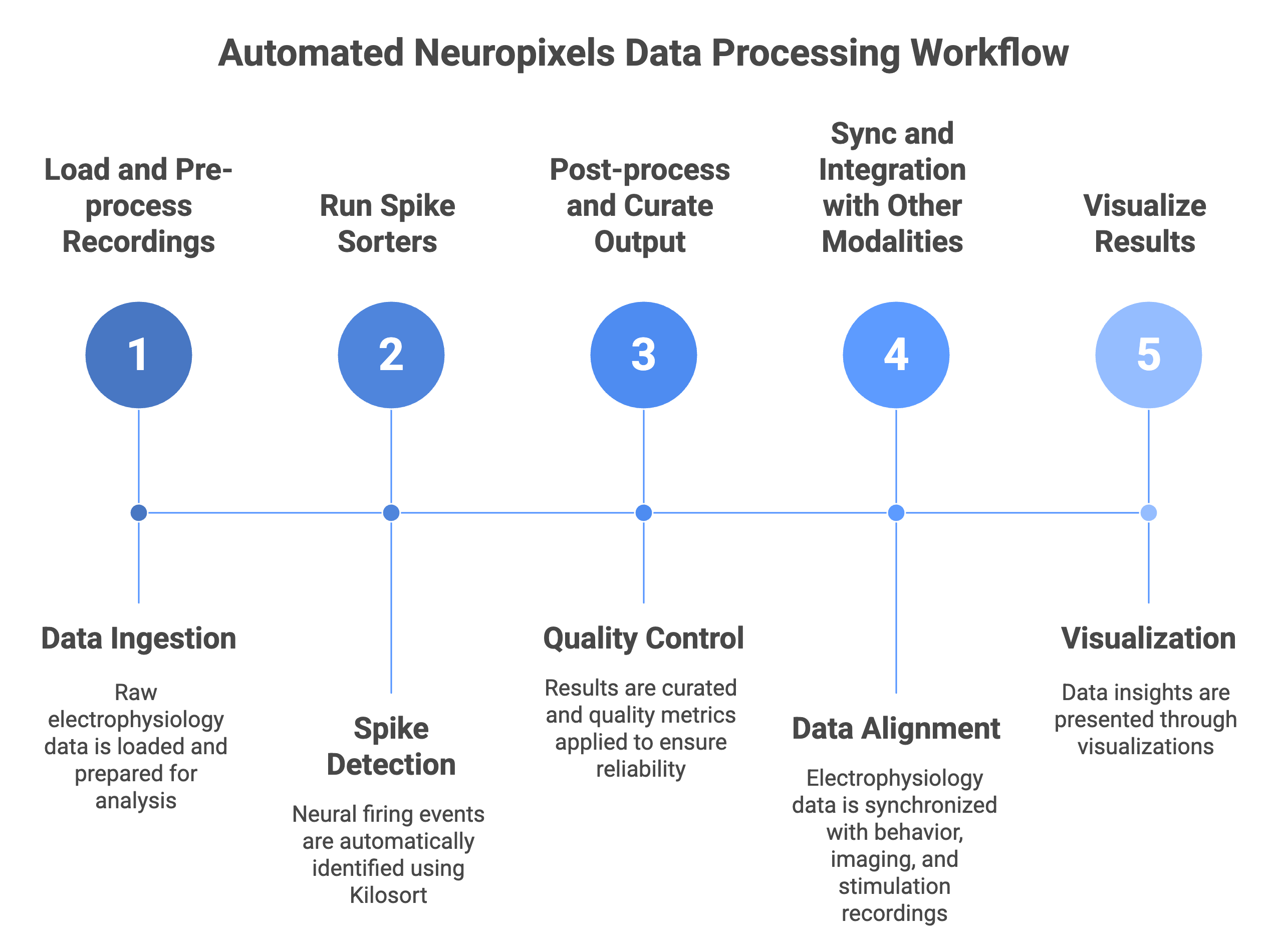
The Element Array Ephys, a component of the NIH U24-funded DataJoint Elements initiative, delivers validated Neuropixels workflows for acquisition and analysis:
- Ingests from SpikeGLX and Open Ephys automatically
- runs spike sorting with Kilosort and other algorithms through SpikeInterface
- Syncs and integrates with behavior, stimulation, and imaging
- Writes results to Neurodata Without Borders (NWB) for sharing
- Stores everything in structured, queryable databases for collaborative work.
Labs apply these approaches to deliver rigorous, reproducible analyses on large datasets. DataJoint helps you spend less time on infrastructure and more time on experiments.
Rigor and Transparency
The DataJoint Platform is a computational environment for scaling modern neuroscience — on the cloud or on premises in research labs. It automates steps from acquisition to sorting and analysis, scales to terabytes, integrates electrophysiology with multiple modalities, including behavior, imaging, and stimulation, and ships as open, community-validated workflows. FAIR practices and NWB export are built in. Additional capabilities include manual spike curation, advanced quality metrics for spikes and units, event-aligned analysis (e.g., PSTHs), and efficient compression of raw data for faster uploads.

Looking Ahead
Neuropixels continue to expand the boundaries of what is experimentally possible. As density and multimodal designs grow, workflows must keep pace. By working with the community and delivering scalable, cloud-based pipelines, DataJoint helps turn rich recordings into reliable, shareable science.
Upload. Analyze. Share. No local infrastructure required.
- Explore Element Array Ephys tutorials: https://github.com/datajoint/element-array-ephys
- Contact us for a demo of DataJoint SciOps for Neuropixels.
- See you at SfN 2025! We're excited to showcase our latest research. Don't miss our nanosymposium presentation titled “Parametric Stimuli Reveal Functional Subcircuits in Visual Cortex.” Be sure to visit our poster, #PSTR198, where we'll present “A Principled Framework for Compression and Standardization of Multiphoton Data." Excited to connect with you there!
* Estimated from PubMed papers mentioning ‘Neuropixels’ since 2017, de-duplicated by institution and counting consortium papers once. This is a conservative lower bound.
References
[1] Jun, J. J., Steinmetz, N. A., Siegle, J. H., et al. (2017). Fully integrated silicon probes for high-density recording of neural activity. Nature, 551(7679), 232–236.
[2] Simons Foundation (2017). 'Neuropixels' expand access to the brain
[3] The Brain Probe Consortium: Neuropixels silicon probes.
[4] Steinmetz, N. A., Koch, C., Harris, T. D., and Carandini, M., et al. (2021). Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science.
[5] Siegle, J. H., et al. (2017). Open Ephys: An open-source, plugin-based platform for multichannel electrophysiology. J Neural Eng, 14(4).
Related posts
AI and the Evolution of Relational Schemas
Insight Entrepreneurship – A New Vision for Science
The Power of Schemas
Updates Delivered *Straight to Your Inbox*
Join the mailing list for industry insights, company news, and product updates delivered monthly.

.svg)